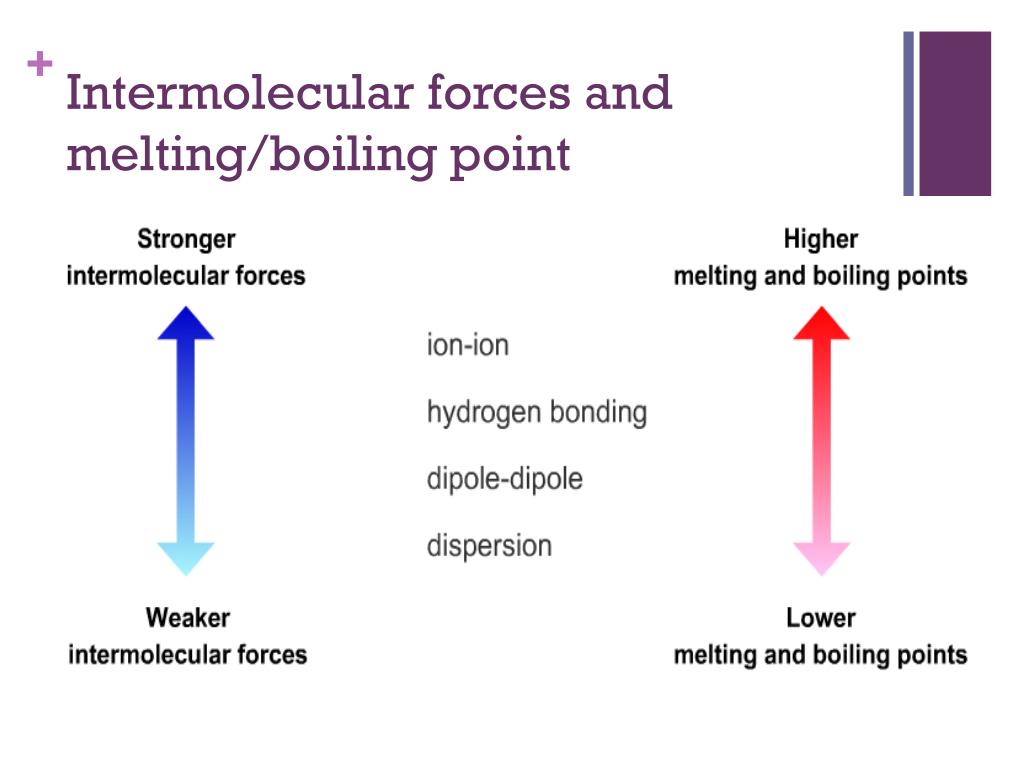
What Effect Does Surface Tension Have On Intermolecular Forces . liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends on the nature of the liquid, the surrounding. one of the effects of intermolecular forces is surface tension. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid.
from www.slideserve.com
This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension depends on the nature of the liquid, the surrounding. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. one of the effects of intermolecular forces is surface tension. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. surface tension is caused by the effects of intermolecular forces at the interface.
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free
What Effect Does Surface Tension Have On Intermolecular Forces This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. one of the effects of intermolecular forces is surface tension. surface tension is caused by the effects of intermolecular forces at the interface. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension depends on the nature of the liquid, the surrounding.
From www.youtube.com
CHEMISTRY 101 Effects of intermolecular forces on surface tension What Effect Does Surface Tension Have On Intermolecular Forces one of the effects of intermolecular forces is surface tension. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. surface tension is caused by the effects of intermolecular forces at. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube What Effect Does Surface Tension Have On Intermolecular Forces liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. one. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free What Effect Does Surface Tension Have On Intermolecular Forces Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. surface tension is caused by the effects of intermolecular forces at the interface. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: This property is caused by. What Effect Does Surface Tension Have On Intermolecular Forces.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and What Effect Does Surface Tension Have On Intermolecular Forces the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension depends on the nature of the liquid, the surrounding. surface tension is caused by the effects of intermolecular forces at the interface. one of the effects of intermolecular forces is surface tension. surface tension, capillary. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Effect Does Surface Tension Have On Intermolecular Forces This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends. What Effect Does Surface Tension Have On Intermolecular Forces.
From byjus.com
Explain the surface tension phenomenon with examples. What Effect Does Surface Tension Have On Intermolecular Forces surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension surface tension. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Effect Does Surface Tension Have On Intermolecular Forces surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. one of the effects of intermolecular forces is surface tension. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. surface tension is caused by the. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation What Effect Does Surface Tension Have On Intermolecular Forces surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. one of the effects of intermolecular forces is surface tension. Surface tension depends on the nature of the liquid, the surrounding. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid. What Effect Does Surface Tension Have On Intermolecular Forces.
From slideplayer.com
Chapter 11 Intermolecular Attractive Forces, Liquids, and Solids ppt What Effect Does Surface Tension Have On Intermolecular Forces surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint What Effect Does Surface Tension Have On Intermolecular Forces Surface tension is the elastic property of the surface of a liquid, such as how water drops form a spherical shape. one of the effects of intermolecular forces is surface tension. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. surface tension surface tension. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint What Effect Does Surface Tension Have On Intermolecular Forces liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Surface tension depends on the nature of the liquid, the surrounding. This property is caused by intermolecular forces, which keep the molecules. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Effect Does Surface Tension Have On Intermolecular Forces surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. surface tension surface tension results from the net inward force experienced by the molecules on the surface. What Effect Does Surface Tension Have On Intermolecular Forces.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Effect Does Surface Tension Have On Intermolecular Forces surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. one of the effects of intermolecular forces is surface tension. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension is caused by the effects. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Effect Does Surface Tension Have On Intermolecular Forces Surface tension depends on the nature of the liquid, the surrounding. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. surface tension is caused by the effects of intermolecular forces at. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Effect Does Surface Tension Have On Intermolecular Forces one of the effects of intermolecular forces is surface tension. surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. Surface tension depends on the nature of the liquid, the surrounding. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Effect Does Surface Tension Have On Intermolecular Forces surface tension is caused by the effects of intermolecular forces at the interface. This property is caused by intermolecular forces, which keep the molecules together and cause the surface of the liquid to resist external forces. liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension is the elastic. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.youtube.com
Surface tension States of matter and intermolecular forces What Effect Does Surface Tension Have On Intermolecular Forces Surface tension depends on the nature of the liquid, the surrounding. one of the effects of intermolecular forces is surface tension. the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: surface tension surface tension results from the net inward force experienced by the molecules on the surface. What Effect Does Surface Tension Have On Intermolecular Forces.
From www.slideserve.com
PPT Ch. 12 Liquids, Solids, and Intermolecular Forces PowerPoint What Effect Does Surface Tension Have On Intermolecular Forces surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular. one of the effects of intermolecular forces is surface tension. surface tension surface tension results from the net inward force experienced by the molecules on the surface of a liquid. Surface tension depends on the nature of the liquid,. What Effect Does Surface Tension Have On Intermolecular Forces.